Translate this page into:
Current status of temporomandibular joint disorders and the therapeutic system derived from a series of biomechanical, histological, and biochemical studies
This article was originally published by Wolters Kluwer and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This article was designed to report the current status of temporomandibular joint disorders (TMDs) and the therapeutic system on the basis of a series of clinical, biomechanical, histological and biochemical studies in our research groups. In particular, we have focused on the association of degenerative changes of articular cartilage in the mandibular condyle and the resultant progressive condylar resorption with mechanical stimuli acting on the condyle during the stomatognathic function. In a clinical aspect, the nature and prevalence of TMDs, association of malocclusion with TMDs, association of condylar position with TMDs, association of craniofacial morphology with TMDs, and influences of TMDs, TMJ-osteoarthritis (TMJ-OA) in particular, were examined. In a biomechanical aspect, the nature of stress distribution in the TMJ from maximum clenching was analyzed with finite element method. In addition, the pattern of stress distribution was examined in association with varying vertical discrepancies of the craniofacial skeleton and friction between the articular disk and condyle. The results demonstrated an induction of large compressive stresses in the anterior and lateral areas on the condyle by the maximum clenching and the subsequent prominent increases in the same areas of the mandibular condyle as the vertical skeletal discrepancy became more prominent. Increase of friction at the articular surface was also indicated as a cause of larger stresses and the relevant disk displacement, which further induced an increase in stresses in the tissues posterior to the disks, indicating an important role of TMJ disks as a stress absorber. In a histological or biological aspect, increase in TMJ loading simulated by vertical skeletal discrepancy, which has already been revealed by the preceding finite element analysis or represented by excessive mouth opening, produced a decrease in the thickness of cartilage layers, an increase in the numbers of chondroblasts and osteoclasts and the subsequent degenerative changes in the condylar cartilage associated with the expression of bone resorption-related factors. In a biochemical or molecular and cellular aspect, excessive mechanical stimuli, irrespective of compressive or tensile stress, induced HA fragmentation, expression of proinflammatory cytokines, an imbalance between matrix metalloproteinases and the tissue inhibitors, all of which are assumed to induce lower resistance to external stimuli and degenerative changes leading to bone and cartilage resorption. Excessive mechanical stimuli also reduced the synthesis of superficial zone protein in chondrocytes, which exerts an important role in the protection of cartilage and bone layers from the degenerative changes. It is also revealed that various cytoskeletal changes induced by mechanical stimuli are transmitted through a stretch-activated or Ca2+ channel. Finally, on the basis of the results from a series of studies, it is demonstrated that optimal intra-articular environment can be achieved by splint therapy, if indicated, followed by occlusal reconstruction with orthodontic approach in patients with myalgia of the masticatory muscles, and TMJ internal derangement or anterior disk displacement with or without reduction. It is thus shown that orthodontic treatment is available for the treatment of TMDs and the long-term stability after treatment.
Keywords
Articular chondrocyte
condylar resorption
extracellular matrix metalloproteinase
mechanical stimuli
temporomandibular joint-osteoarthritis
INTRODUCTION
Temporomandibular joint (TMJ) consists of hard and soft tissues. Hard tissue is composed of mandibular condyle and glenoid fossa. Meanwhile, soft tissue comprises articular disk, ligament, synovium, and masticatory muscles. It should be noted that synovial joints with articular disks are only temporomandibular, knee and scapuloclavicular joints in humans. Furthermore, the following functional features are found in TMJ.
Class III fulcrum: Masticatory muscles act on the dentition, and the residual stress is loaded on the TMJ. Thus, TMJ is accepted as a load-bearing organ.
Translatory movement available only in TMJ: Translatory repositioning of the condyle is available only in TMJ in addition to the pure rotation, but not induced in other general joints.
Cooperative movement of the condyle and disk: Articular disk located between the articular surface of the condyle and glenoid fossa experiences a cooperative movement with condylar repositioning during mouth opening/closing. Such behavior of the disk exerts a crucial role in stress absorbing.
Temporomandibular joint disorder (TMD) has become an important disease in dentistry and/or orthodontics. Under such background, various studies have been conducted to elucidate the nature and causes of TMD in association with various etiologic factors.[1-4] As a result, TMD is currently accepted as a multi-factorial disease, however, occlusal parameters have also been speculated to have a certain association with TMD.[5,6]
In this article, clinical aspect of TMDs is first discussed with a reference to the relevant clinical parameters key to the onset of TMDs. Then, in order to elucidate the association of various clinical parameters with the nature of stresses acting on the TMJ structures, finite element stress analyses are introduced. Biological and histological changes in the condylar cartilage are then examined when excessive mechanical stimuli are loaded to the mandibular condyle. Finally, in a biochemical aspect, molecular and cellular changes are investigated for cell morphology, amount of extracellular matrices, matrix metalloproteinases (MMPs) and superficial zone protein (SZP) as a protector of the cartilage and subchondral bone when various mechanical stimuli are applied to cultured chondrocytes.
CLINICAL PARAMETERS RELEVANT TO TEMPOROMANDIBULAR JOINT DISORDERS
We would like to first review a series of studies on TMDs and the relevant factors for better understanding the nature of TMDs. These subjects are:
The nature and prevalence of TMD,
Association of malocclusion with TMD,
Association of condylar position with TMD,
Association of craniofacial morphology with TMD, and
Influences of TMD, TMJ-osteoarthritis (TMJ-OA) in particular, from a clinical viewpoint.
First, prevalence of TMD was examined in an orthodontic patient group. In this survey, the percentage of TMD patients to the total number of patients was approximately 14%.[7] It is surprising to know very high prevalence of TMDs, which are mostly occupied by TMJ internal derangement with various intra-articular pathologic stages, in adolescent patients with malocclusion. It is also of a clinical significance that the adult population has a higher prevalence,[8] and jaw deformity patients exhibit substantially higher prevalence[9] than adolescent patient group and asymptomatic adult volunteers, respectively. Furthermore, it is of a great interest that the prevalence of TMJ-OA is about 18% in all the TMD patients and approximately 2.5% in all the patients.[7]
The second topic is the association of malocclusion with TMDs. The prevalence of TMD was considerably higher in open bite, deep bite, and posterior cross-bite.[10] Thus, some specific types of malocclusion were significantly associated with the occurrence of TMD in patient group. It is also speculated from this finding that condylar displacement in the TMJ space may change disk position relative to the displaced condyle and result in the onset of TMJ internal derangement.[11,12]
The next topic is the association between condylar position in the TMJ space and intra-articular pathologic status. Condylar position was more posterior in anterior disk displacement with reduction (AWWD), whereas concentric in anterior disk displacement without reduction (ADDWo).[11] It is thus indicated that condylar position is directly relevant to the disk displacement and the nature of TMJ internal derangement or the progress in intra-articular pathologic status from ADDW to ADDWo.[2,4,11]
Then, the association between craniofacial morphology and intra-articular pathologic status was examined by means of the Spearman’s rank correlation analysis. The size and position of the mandible presented significantly negative correlations and the mandibular plane and ANB angles exhibited significantly positive correlations with the pathologic stages [Figure 1].[7,13,14] In addition, as a result of correlation analysis for lateral shift of the mandible and intra-articular pathologic stages defined by Wilkes,[14] the amount of mandibular lateral shift presented a significantly positive correlation with the pathologic status of TMDs [Figure 2], indicating that TMDs produce less growth of mandible bilaterally or unilaterally when affected on both sides or one side. It is thus demonstrated from these results that the progress in intra-articular pathologic status is highly related to more severe discrepancy of the craniofacial skeleton in the vertical direction or more prominent lateral shift of the mandible in the transverse direction.
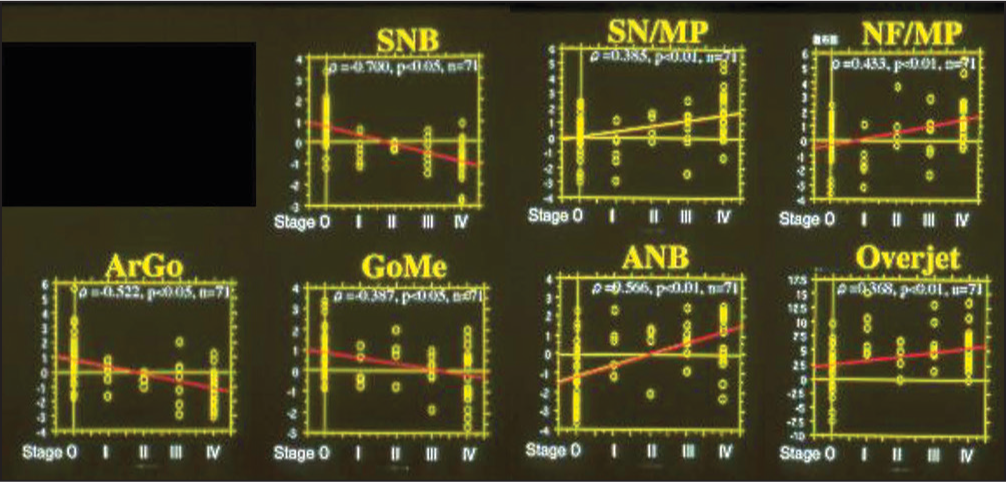
- Association between morphology of the craniofacial skeleton and intra-articular pathologic stages, defined by Wilkes, in patients with malocclusion
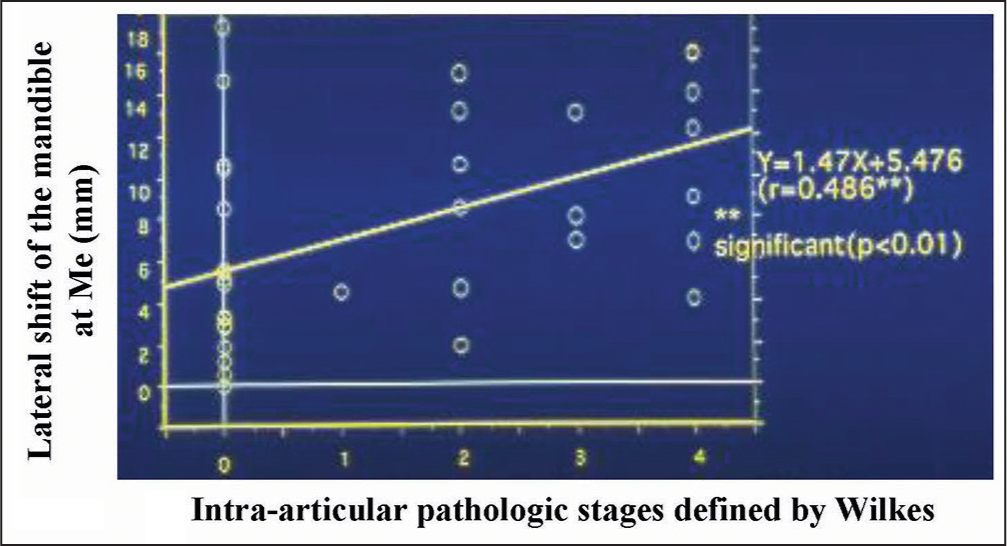
- Association between lateral shift of the mandible with intra-articular pathologic stages, defined by Wilkes, in adult patients with jaw deformity
As the influences of TMJ-OA on orthodontic treatment and the outcomes, the morphometric findings mentioned above and the relevant actual TMJ-OA cases provide us with very interesting and useful clinical implications such that condylar resorption in TMJ-OA produces jaw deformity with less developed and distally located mandible and affects the outcomes and stability of occlusal reconstruction.[1,7,9,15,16] Figures 3-6 show a case of TMJ-OA appeared during retention after orthodontic treatment. She exhibited open bite with Class II molar relation [Figure 3]. On the lateral cephalogram and in the analysis, distally-located and more divergent mandible with less developed condyle due to condylar resorption was revealed [Figure 4]. This case was first treated with functional appliance and then followed by edgewise technique under non-extraction [Figure 5]. However, during the retention, open bite was induced by progressive condylar resorption (PCR), indicating that TMJ-OA affects orthodontic treatment and the outcomes substantially [Figure 6].
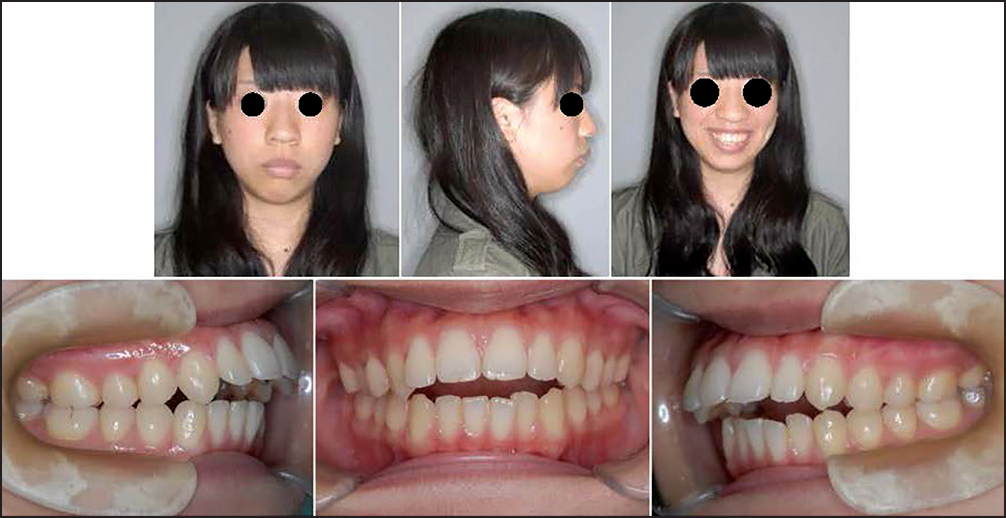
- Jaw deformity patient with distally-located mandible due to progressive condylar resorption after orthodontic treatment
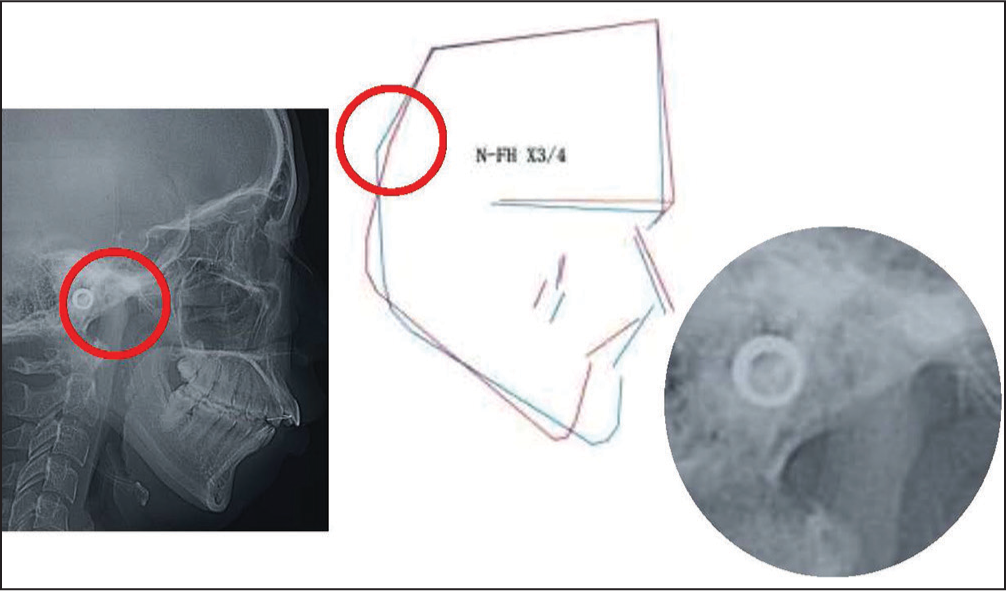
- Lateral cephalogram and the analysis a red circle shows the condyle, which is enlarged on the right. It is prominent that the condyles exhibit resorption at the anterior surface, leading to a mesial shift of “Ar” and the resultant characteristic inclination of the mandibular ramus, which is common to patients with condylar resorption from temporomanidular joint-osteoarthritis
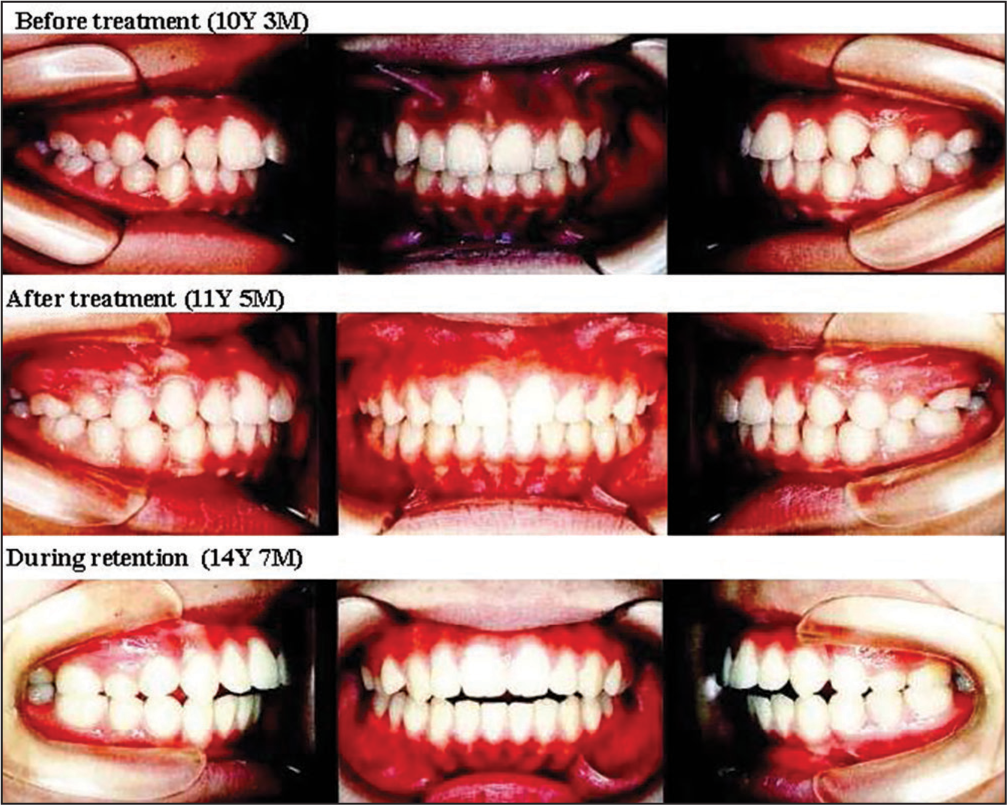
- Changes in occlusion before and after orthodontic treatment and during retention
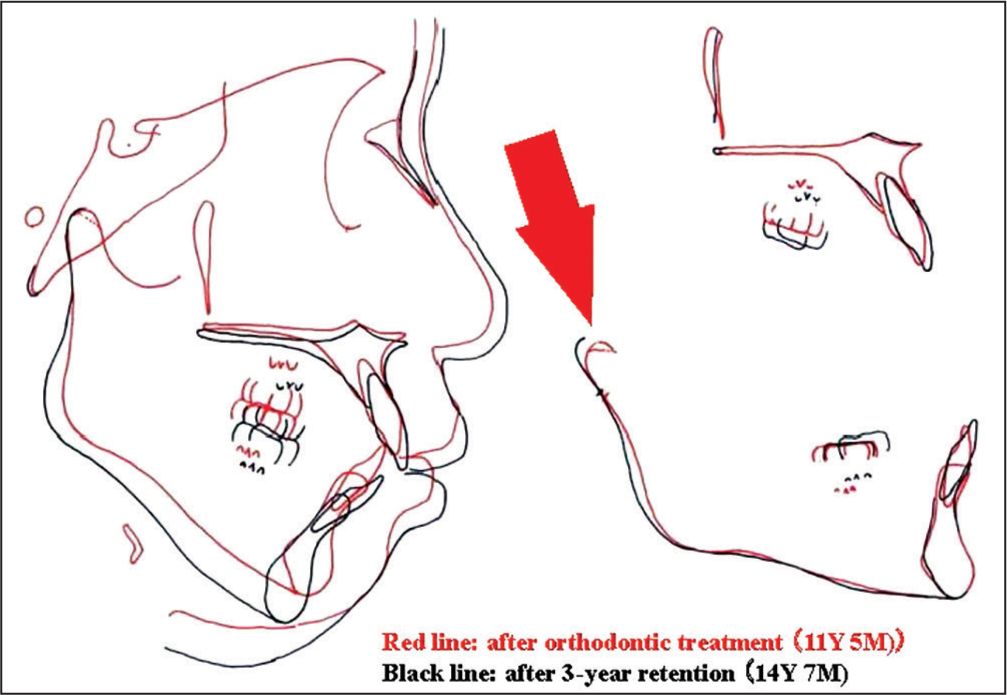
- Changes in the position of mandible after orthodontic treatment due to progressive condylar resorption a red arrow indicates an existence of condylar resorption at the anterior surface of condyle during retention. An existence of condylar resorption at the anterior surface of condyle during retention
On the contrary, it is demonstrated in the treatment cases of TMJ-OA that the sable occlusion achieved by orthodontic occlusal reconstruction has produced biomechanical equilibrium in the TMJ and subsequently provided the condyle with a potential for adaptive or functional remodeling.[17] The details will be shown later in the final part of this article.
These findings are very useful for understanding the nature of TMDs and the mechanisms of degenerative changes in condylar cartilage, which may be hypothesized reasonably that various morphological and functional parameters produces an increase in TMJ loading, which further leads to degenerative changes in the articular cartilage of the mandibular condyle and resorption of bone and cartilage expressed as a TMJ-OA.
To demonstrate the above hypothesis, the association between degenerative changes of articular cartilage and mechanical stimuli was examined by a series of studies with biomechanical, histochemical and biochemical approaches.
BIOMECHANICAL CONSIDERATION FOR THE MECHANISMS OF DEGENERATIVE CHANGES IN CONDYLAR CARTILAGE
Temporomanidular joint loadings from maximum clenching
There have been various studies on TMJ loading by use of finite element stress analysis.[18-24] Among these studies, a report by Tanaka et al.[23] can be cited as a representative and frontier study for TMJ loading in the field of biomechanics. In this study, a three-dimensional model of the mandible, including the TMJ was constructed for stress analysis with finite element method [Figure 7]. For loading conditions, the magnitude of muscle forces was determined to exert a resultant force of 500 N, simulating the maximum clenching. Large compressive stresses were induced in the anterior, middle and lateral regions, whereas tensile stresses were found in the remaining areas [Table 1]. In particular, the greatest compressive stresses were found in the anterior region of condyle.[23]
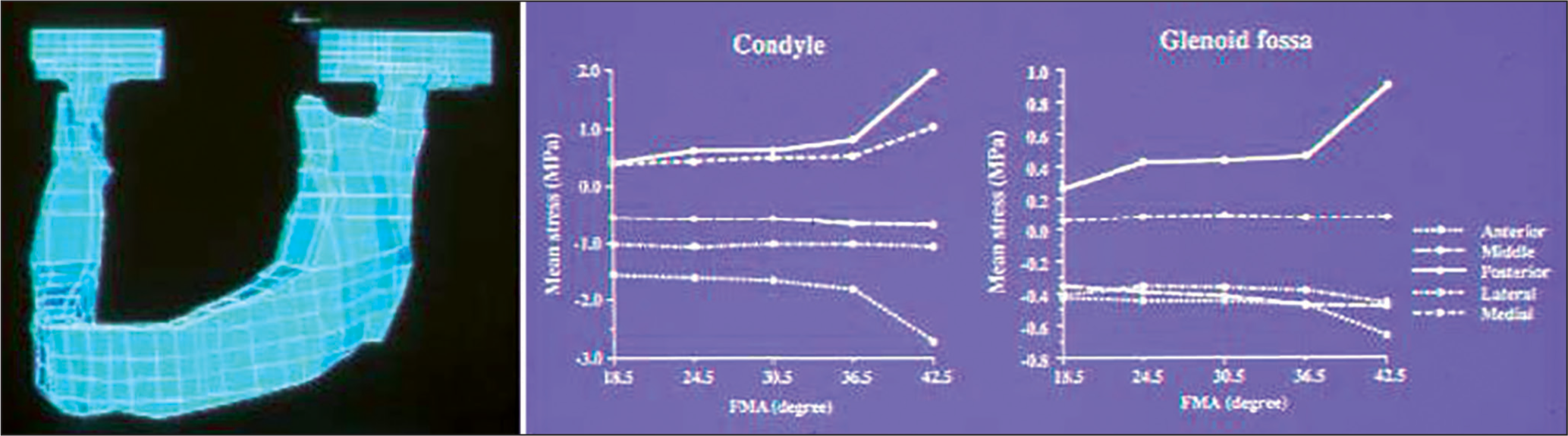
- A three-dimensional finite element model of the mandible including the temporomanidular joint (left) and changes in stresses in the condyle and glenoid fossa in association with vertical skeletal discrepancy simulated by varying FMAs (right)
| Anterior | Middle | Posterior | Lateral | Mesial | |
|---|---|---|---|---|---|
| Condyle | −1.642 | −0.543 | 0.664 | −1.017 | 0.521 |
| Glenoid fossa | −0.440 | −0.410 | 0.445 | −0.351 | 0.103 |
| Articular disk | −0.403 | −0.200 | 0.258 | −0.342 | 0.041 |
TMJ – Temporomanidular joint
The results may be compared with previous anatomic and experimental findings. Oberg et al.[25] demonstrated in a study with cadavers that erosion and ruggedness of the bony structures and thinning and/or perforation of the articular disk were more frequently observed in the anterior and lateral areas of the TMJ. Kopp[26] also reported a higher concentration of glycosaminoglycan, which is regarded as a marker of compression, in the anterior and lateral areas of the TMJ. Thus, the greater compressive stresses in the anterior and lateral areas revealed in the above study may account for the various degenerative changes in the articular disk and condyle reported in the anatomic studies[25,26] and clinical cases of TMJ-OA.[1,7,15-17]
Temporomandibular joint loadings varied by vertical skeletal discrepancy
In previous morphometric and radiographic studies,[7,9,13,15] vertical discrepancy was revealed as a key determinant for the intra-articular pathological status of TMD. Thus, the stress distributions were analyzed in association with the discrepancy with an assumption that such morphologic parameter affects the nature of TMJ loading.[24] For stress analysis, the model developed above was modified to represent vertical discrepancies of the craniofacial complex by changing the shape of mandible, maintaining the number of nodes and elements in the standard model. The gonial and mandibular plane angles were changed with a special reference to the means and standard deviations. These stresses were changed in association with varying mandibular plane angles and exhibited more substantial changes than those with varying gonial angles.[24] Changes in the stresses were nonlinear in nature and particularly drastic when the angle became larger than a certain threshold value [Figure 7].
It is thus demonstrated that vertical skeletal discrepancy induces an increase in TMJ loading and lack in biomechanical equilibrium for the TMJ components. These results may help explain the finding that malocclusions with vertical discrepancies have a higher prevalence of TMD than others.[5,10] This may be due to the malposition of condyle in the glenoid fossa in TMD cases with vertical discrepancies.[10-12] Another explanation is that a lack in biomechanical equilibrium in the TMJ induced by the skeletal discrepancies may produce nonlinear and plastic deformation of the articular disk observed in a tensile test of the disk[27] and more extensive degenerative changes in the mandibular condyle with articular cartilage. These speculations are to be examined later in terms of biologic and biochemical responses of articular cartilage by in vivo and in vitro experiments.
Association of friction at the articular surface with temporomandibular joint loading and disk displacement
During mouth opening, condylar movement essentially requires cooperative disk motion. When the disk slides on the articular surface, shear stress is induced but negligible because of the very low friction.[28] Furthermore, the presence of synovial fluid decreases the frictional coefficient to almost zero.[29] Meanwhile, under various pathological conditions with intra-articular inflammation and degradation of hyaluronan (HA), an increase in friction at the articular interfaces is generated eventually.
Given these considerations, a biomechanical study with finite element analysis was conducted to assess the stress distribution and disk displacement during mouth opening in association with different frictional coefficients. It is demonstrated that an augmentation in the friction at the articular surface produces an increase in stresses and the subsequent disk displacement, leading to the onset of TMJ internal derangement or the more progressed form with degenerative changes.[30] Changes in stress distribution in the TMJ were also reported in previous studies by Foster and Fisher[29] and Williams et al.[31] It is also indicated that the amount of friction is increased proportionally to the magnitude and duration of TMJ loading, relating in part to the onset of disk displacement.[32]
Thus, friction at the articular surface is a key determinant for maintaining optimal intra-articular environment which allows smooth movement of the condyle. Therefore, it is of a great significance to eliminate excessive friction at the interfaces between TMJ structures. To this end, various lubricants equivalent to the synovial fluid has been used not to make progress in intra-articular pathology. HA addition, as an application of lubricant, will be introduced in the following section.
Friction at the articular surface and lubrication function
When the TMJ disk slides along the articular surfaces during jaw movement, shear loading of the disk can be considered to be negligible, due to very low friction.[28] Friction in synovial joints, in general, is associated with its lubrication mechanism,[33] which in turn is dependent on the rheological properties of synovial fluid.
Hyaluronan (HA), which occupies 0.14-0.36% of synovial fluid in normal subjects,[34] is one of the principal components determining its rheological properties. The amount of viscosity, an essential determinant for the lubrication function, is dependent on the molecular weight.[35] In joints affected with OA, meanwhile, the synovial fluid has a reduced viscosity due to a decline in both concentration and molecular weight of HA.[36]
Given these findings, Kawai et al.[37] examined the role of HA in the lubrication of the TMJ. They measured the frictional coefficients in the porcine TMJ after the application of HA with different molecular weights and concentrations. Application of HA resulted in a significant decrease in the frictional coefficient by 50-70%. This study thus reached a conclusion that the addition of HA did reduce the coefficient of friction under the experimental conditions, supporting an assumption that the most superficial layer plays a significant role in the adequate lubrication function, which apparently can’t be established by the physical action of cartilage alone but by a cooperative function of various TMJ components.[37]
Role of the articular disk in buffering or absorbing stresses on the temporomandibular joint components
It is well understood that TMJ is one of load-bearing organs in the human body, and the disk plays an important role as a stress absorber, resulting in stress reduction and redistribution in the joint.[38-40] For maintaining optimal intra-articular environment, therefore, two major factors can be indicated; that is healthy disk and optimal positional relation between the disk and bony components.
Mechanical behavior of the disk has already been examined extensively. The elastic and viscoelastic features are described by various parameters such as elastic modulus, instantaneous modulus, relaxed modulus and the strain-relaxation time.[27,41-43] In addition, viscoelastic material model was examined for TMJ disk, demonstrating that a four-mode Maxwell model is more suitable for representing mechanical behavior of the disk during stress absorption.[44]
Furthermore, Tanaka and van Eijden[45] published a review about the fundamental concepts of the biomechanical behavior of the TMJ disk. In conclusion of the review, they described that the TMJ disk behaves as a viscoelastic structure and can function as a stress absorber and distributor. This means a biomechanical contribution of the disk to prevent stress concentration and excessive stress in the cartilage and bony components of the joint, all of which protect the joint from degenerative and osteoarthritic changes. They also discussed the disk wear in the anterior[26] and intermediate[46] regions. As mentioned in the preceding sections in this article, these phenomena have already been explained in association with excessive loading validated by finite element stress analyses[18-20,23] and histological or histochemical examination which revealed stress concentration by the presence of chondroitin sulfate.[47,48] In addition, it was demonstrated in a previous study[21] that stresses in the retrodiscal tissues were increased by anterior disk displacement and may lead to the thinning and perforation, indicating an important role of the disk as a stress absorber. It is also of a great significance to keep it in mind that the mechanical properties are varied by various intrinsic and extrinsic factors such as aging, trauma and pathologic disease.[45]
Recently, we have examined if the breakdown of joint lubrication affects mandibular condylar cartilage’s frictional property and leads to subsequent degenerative changes in TMJ.[49] The frictional coefficient was measured in porcine TMJ by a pendulum device after digestion with hyaluronidase (HAase) or trypsin. Gene expressions of interleukin-1 β (IL-1 β), cyclooxygenase-2 (COX-2), MMPs, type II collagen and histology were examined after prolonged cyclic loading by an active pendulum system. The results showed that the frictional coefficient increased significantly after HAase (35%) or trypsin (74%) treatment. Gene expression of IL-1 β, COX-2 and MMP-1, 3 and 9 increased significantly in enzyme-treated TMJs after cyclic loading. The increase was greater in trypsin-treated group than in HAase-treated group. Type II collagen expression was also reduced in both enzyme-treated groups. Histology revealed surface fibrillation and increased MMP-1 in trypsin treated group, as well as increased IL-1 β in both enzyme-treated groups after cyclic loading. These findings have demonstrated the compromised lubrication in TMJ is highly associated with altered frictional properties and surface wear of condylar cartilage, accompanied by the release of proinflammatory and matrix degradation mediators under mechanical loading.[49]
BIOLOGICAL AND HISTOLOGICAL CONSIDERATION FOR THE MECHANISMS OF DEGENERATIVE CHANGES IN CONDYLAR CARTILAGE
Histochemical changes in the condylar cartilage in response to excessive temporomandibular joint loading resulted from simulated vertical skeletal discrepancy
In the preceding section, various biomechanical studies have shown the presence of excessive or imbalanced mechanical stresses on the TMJ structures. Furthermore, it should be noted that TMJ internal derangement with degenerative changes may be relevant to such morphologic characteristics of the mandible as steep mandibular plane, and short ramus expressed as vertical skeletal discrepancies. Therefore, biological responses of condylar cartilage to enhanced TMJ loading, produced by vertical skeletal discrepancy simulated in biomechanical analysis, are discussed herein with a special reference to the remodeling of cartilaginous tissues on the mandibular condyle.
First, an in vivo study with histochemical and morphometric approaches is introduced.[50] They simulated vertical skeletal discrepancies in 4-week-old rats by use of a 1 mm - thick metal plate bonded onto the maxillary molars [Figure 8]. In fact, a backward and downward rotation of the mandible was confirmed on serially taken lateral cephalograms, indicating an increase in the TMJ loading on the condyle. The tissue sections were stained with tartrate-resistant acid phosphatase (TRAP) and hematoxylin and eosin for histomorphometric analyses of the thickness of cartilage layers and the number of TRAP-positive cells. During the initial phase of the experiment, the thickness of proliferative and hypertrophic zones in the anterior and superior regions of the condyle was significantly smaller than in the control. The number of TRAP-positive cells was significantly greater in the experimental group than in the controls at the initial phase of the experiment [Figure 9]. Morphometric analyses revealed less-developed mandible, decreased ramus height and large gonial angle in the experimental group [Figure 10]. From these findings, it is shown that biomechanical changes in the intra-articular environment associated with vertical skeletal discrepancy influences or inhibits cartilaginous growth of the condyle and mandible to a considerable extent if induced during growing period.[50]
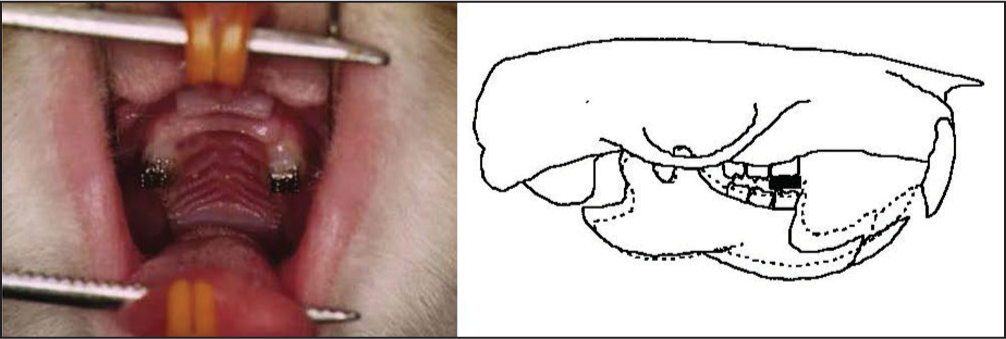
- An experimental appliance (left) to induce backward and downward displacement of the mandible (right) and the resultant increase in stresses acting on the condyle, which is demonstrated in the preceding finite element analysis

- The number of tartrate-resistant acid phosphatase-positive cells, key to condylar resorption, in the anterior (left) and superior (right) cartilage layers on the mandibular condyle

- Changes in the size and shape of mandible produced by simulated vertical skeletal discrepancy and the resultant increase in mechanical stresses acting on the condyle
Various studies have shown that responses of the condylar cartilage may be altered by changes in the biological and biomechanical environments in the TMJ space.[51-53] Similar changes were demonstrated in experimental animals when the incisors were trimmed or removed.[51] McNamara[54] investigated the influences of increases in the vertical dimension on craniofacial adaptation in growing monkeys, demonstrating that the amount of vertical growth at the condylar head was decreased, which is assumed due to reduced cartilaginous remodeling and growth. From these studies, it is emphasized that mechanical stimuli acting on the condyle surely reduce cartilaginous remodeling, if excessive, leading to a decrease in the growth of overall condyle and mandible, which in turn produces vertical skeletal discrepancies with small mandible. Subsequently, such morphologic characteristics would produce enhanced TMJ loading and degenerative changes in the TMJ components.[50]
Changes in the condylar cartilage and masticatory muscles from excessive temporomandibular joint loading due to forced mouth opening
Another in vivo experiment is introduced herein to examine pathological changes in the condylar cartilage from excessive mouth opening, which is speculated to generate an increase in TMJ loading on the condyle and glenoid fossa, and the subsequent masticatory muscle disorders. Kawai et al.[55] examined the association of mechanical loading with the induction of OA-lesion in the rat TMJ and its influence on jaw muscle activity. Mechanical stress was applied to the rat TMJ by forced mouth opening of 3 h/day for 5 days. As a result, the condylar cartilage exhibited OA-like lesions with a decrease in the number of chondrocytes immediately after the experiment. Immediately after the beginning of forced mouth opening, the total duration of muscle activity, defined as a duty time, increased significantly in the masseter muscle, whereas decreased significantly in the digastric muscle at the low activity level. These results suggested that mechanical overloading to the TMJ induced OA-like lesion and the intra-articular pathological status influenced the nature of jaw muscle activity, at a low activity level in particular.
Similar study was conducted in growing rats subjected to forced mouth opening to increase mechanical stress on the mandibular condyle.[56] As a result, marked OA-like lesions were observed in the condyle. In addition, vascular endothelial growth factor (VEGF) was detected in the chondrocytes of the mature and hypertrophic cell layers of the intermediate and posterior regions of the condyle. The percentage of VEGF immune-positive chondrocytes significantly increased with longer application of excessive TMJ loading. Furthermore, TRAP staining of the condylar cartilage showed a significant increase in the number of osteoclasts in the mineralized layer subjacent to the hypertrophic layer where high VEGF expression was detected, suggesting an important role of VEGF in the progression of TMJ-OA.[56]
These findings were also found in previous studies,[57,58] demonstrating the expression of VEGF with an ability to induce osteoclasts in association with excessive mechanical stress. Freemont et al.[59] reported that VEGF expression in chondrocytes is induced by high-intensity stress and acts in cartilage as an autocrine inducer of MMPs. Furthermore, Forsythe et al.[60] described that VEGF induction in chondrocytes by excessive mechanical stimuli is linked to activation of the hypoxia-induced transcription factor-1 (HIF-1), which is well known to bind to hypoxia response element in the human VEGF gene promoter. It may be a conclusion from these studies that VEGF is probably induced in chondrocytes by excessive mechanical stimuli, facilitating hypoxia to mediate degenerative or destructive processes with the induction of MMPs as an autocrine factor.[56]
BIOCHEMICAL CONSIDERATION FOR THE MECHANISMS OF DEGENERATIVE CHANGES IN CONDYLAR CARTILAGE
Biological and biochemical responses of articular chondrocytes to excessive mechanical stress
Articular cartilage contains a large amount of matrix macromolecules such as proteoglycan and type II collagen. These molecules contribute to the flexibility of cartilage and protection of the joint components from various mechanical stimuli. It is indicated that the mechanical load of appropriate magnitude is essential for the growth and differentiation of chondrocytes. On the other hand, excessive loads influence harmfully the articular cartilage and induce various degenerative joint diseases.[61] It is suggested that excessive or imbalanced mechanical loads induce deformation of the articular cartilage and the subsequent degradation of the cartilage matrices. Jeffrey et al.[62] reported a loss of matrix components depending on the degree of mechanical stimuli when articular cartilage biopsy samples were subjected to a single impact load.
In order to elucidate the mechanisms of degradation of the cartilage matrix, various studies have been conducted and demonstrated that proteolytic enzyme, MMP, is a major factor to degrade the macromolecules of connective tissue matrices at neutral PH.[63,64] A lack in balance between MMPs and the tissue inhibitors, TIMPs, was indicated as a cause of matrix degradation.[65] Proinflammatory cytokines such as IL-1beta and TNF-alpha were also demonstrated to have a close relation to the expression of MMPs. [59,66,67] In addition, HA fragmentation or reduced HA synthesis, induced by HAase and inflammatory cytokines respectively, was demonstrated as an important event for pathologic and degenerative changes in chondrocytes and synoviocytes.[68-71]
Furthermore, Honda et al.[72] designed a series of studies of biological and biochemical responses of articular chondrocytes to an excessive tensile stress. Chondrocytes, isolated from the knee joint cartilage of 4-week-old rabbits, were subjected to a high magnitude tensile stress of 17 kPa at a frequency of 30 cycles/min for 12 h or 24 h [Figure 11]. They examined the protein levels of cartilage matrices and the gene expressions of MMPs, TIMPs, and proinflammatory cytokines. A change in cell morphology from a polygonal to spindle-like shape was observed. Toludine blue staining, type II collagen immunostaining, and an assay of the incorporation of [35S] sulfate into proteoglycans revealed a decrease in the level of cartilage-specific matrices such as type II collagen and HA in chondrocyte cultures [Figures 12 and 13]. Furthermore, the cyclic tensile stress increased mRNA levels of MMP-1, 3, and 9, IL-1 beta, TNF-alpha and TIMP-1 in the cultured chondrocytes [Figure 14], whereas the levels of MMP-2 and TIMP-2 were unchanged.[72]

- Vacuum-induced tensile stress loading to cultured articular chondrocytes

- Changes in the synthesis of type II collagen in cultured articular chondrocytes in loading with excessive tensile stresses

- Changes in the level of hyaluronan in cultured articular chondrocytes in loading with excessive tensile stresses
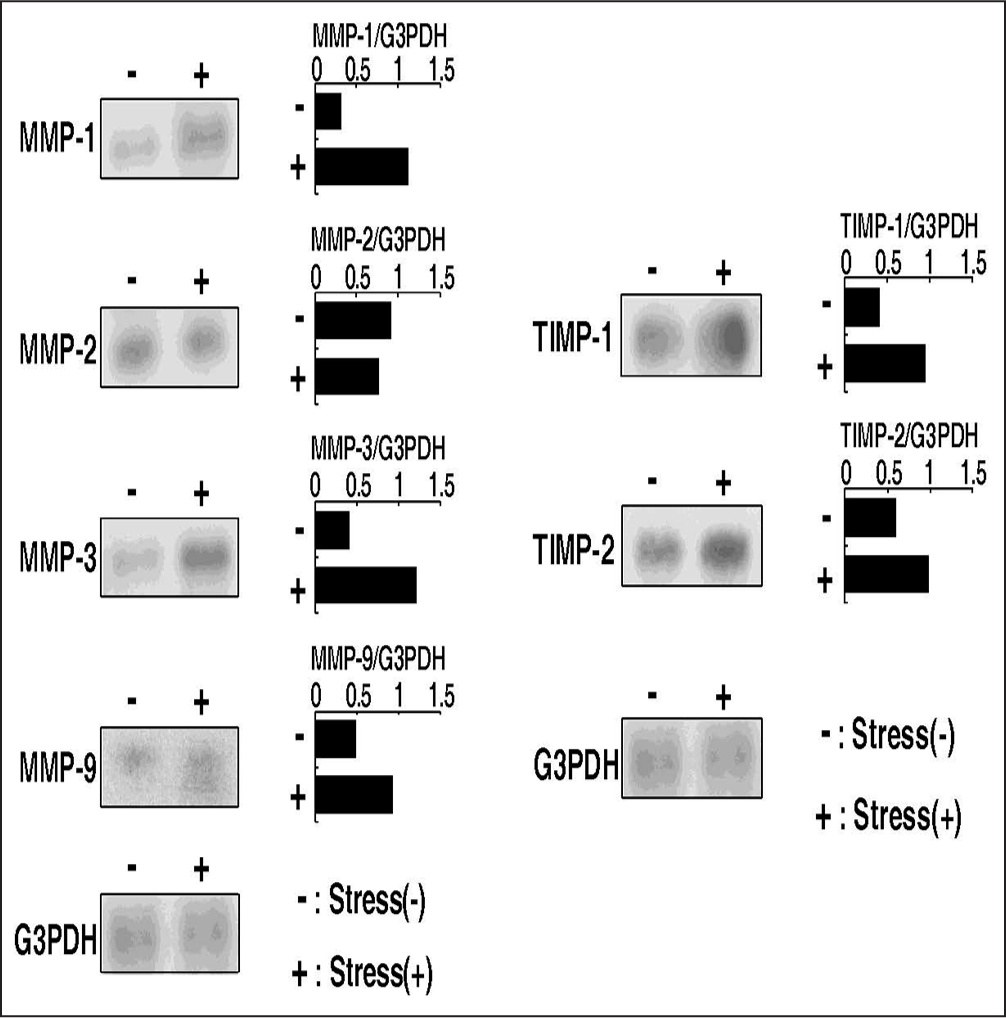
- Influences of excessive tensile stresses on mRNA levels of matrix metalloproteinases and TIMPs in cultured articular chondrocytes
Malek and Izumo[73] reported that endothelial cells in loading with fluid flow stress changed to spindle shape and aligned in the flow direction. The mechanism is also assumed to be dependent on tyrosine kinase activity, intracellular calcium, and an intact microtubule network. It is thus suggested that the stretched load may affect the cell morphological change, but not affect the cell alignment. With respect to the expression of MMPs, TIMPs and proinflammatory cytokines, cytoskeletal deformation induced by cellular changes in morphology was indicated.[74] Furthermore, the cytoskeletal deformation was indicated to be associated with regulation of the gene expression for ECM properties.[75] Meanwhile, it is speculated if the expression of MMPs is a direct effect of excessive mechanical stimuli or an indirect effect through the stimulation of various cytokines such as IL-1 beta and TNF-alpha. With respect to this question, Honda et al.[72] derived an interesting finding that the gene expression of MMP-1, 3, and 9 was observed in the loaded cultures treated with cycloheximide, indicating that the induction of MMP mRNA is not pertinent to the stimulation of cytokines and other inflammatory products. It is also reported that the gene expression of MMP-1, 3, and 9, but not MMP-2 is regulated by the activation of protein kinase C (PKC) in vascular smooth muscle or chondrocytes[76,77] and that a cyclic tensile load activates PKC in chondrocytes.[77]
Furthermore, Ohno et al.[78] detected an existence of superficial zone protein (SZP), a protector for articular cartilage against excessive mechanical stimuli, in the mandibular condyle. In addition, it is shown that the amount of SZP is reduced by excessive mechanical stimuli acting on the cultured chondrocytes,[79] indicating an important role of SZP in the prevention of degenerative changes in the cartilage and the subsequent onset of progressive condylar resorption (PCR) frequently observed in clinical orthodontics, in female patients in particular.
It is thus shown that excessive stresses induce changes in cartilage cell morphology, reducing a synthesis of SZP and cartilage matrices such as type II collagen and proteoglycan, leading to lower resistance of cartilage tissues to external stimuli. Direct effects of the excessive mechanical stress were observed for the induction of MMPs even in the absence of protein synthesis through the recognition of cytoskeleton and activation of PKC following cellular changes in shape. Finally, it is demonstrated that induction of MMPs and proinflammatory cytokines and quantitative imbalance between MMPs and TIMPs directly produce the destruction of cartilage matrices leading to bone or cartilage resorption in the mandibular condyle.
Effects of mechanical stimuli with different frequencies on the metabolism of chondrocytes
In the preceding discussion, the influences of excessive mechanical stimuli on the metabolism of articular cartilage have been well documented with the speculated mechanisms. The remaining parameters of mechanical stimuli, that is compressive or tensile, static or dynamic, high or low frequency, and so on, have to be taken into considerations with a special reference to biologic and cellular responses.
Mechanical loading is classified into static and dynamic ones by its frequency. Various studies conducted using cartilage tissue explants have demonstrated that sustained loading induced the reduction of cartilage metabolism,[80,81] whereas intermittent mechanical stimulation enhanced cartilage metabolism irrespective of the loading type, tension, and compression.[82-84]
From these considerations, influences of loading frequency on the metabolism of chondrocytes was examined.[85] Intermittent tensile stress upregulated the syntheses of DNA and proteoglycan in chondrocytes at the proliferating stage and matrix-forming stages, respectively. Especially, an intermittent tensile stress with 30 cycles/ min or the higher frequencies increased significantly the chondrocyte metabolism. Intermittent compressive stress also significantly enhanced the syntheses of DNA and proteoglycan in chondrocytes, whereas sustained compression significantly decreased these syntheses. These findings suggest that the proliferation and differentiation of growth plate chondrocytes are regulated by the mechanical loading and that the chondrocyte metabolism is enhanced with an increase in the frequency.
One possible mechanism for the cell recognition about mechanical stimuli may be explained by a fact that changes in the level of intracellular calcium ions ([Ca2+ ]i) is caused by the fluid flow,[86] which may be generated by cyclic loading and release of osmotic pressure to the collagen gel. Therefore, intermittent stresses might induce the intra- or extra-cellular Ca2+ mobilization, resulting in the promotion of chondrocyte metabolism.
In conclusion of this section, the metabolism of chondrocytes is significantly enhanced by the intermittent mechanical stress even if the magnitude is lower, suggesting that the frequency of mechanical stress may be the most important factor for modulating the metabolism of chondrocytes.
Signal transmission of mechanical stimuli through cell surface ion channels
Association of biologic and cellular changes with mechanical stimuli has been well documented in the preceding sections. Therefore, based on an evidence that mechanical stress regulates chondrocyte proliferation and differentiation via some cell surface ion channels,[87] Tanaka et al.[88] examined if a specific ion channel is involved in the cascade during the induction of PTHrP by mechanical strain. Cyclic mechanical strain, applied to rat growth plate chondrocytes at a frequency of 30 cycles/min, significantly increased PTHrP mRNA levels in chondrocytes. The induction of PTHrP was inhibited by nifedipine, a Ca2+ channel blocker, but not by the blockers of stretch-activated (S-A) channel.
Yellowley et al.[89] showed that the fluid flow caused mobilization of intracellular Ca2+ in articular chondrocytes by the activation of G-protein. Furthermore, it was reported that either intra- or extra-cellular Ca2+ mobilization was highly associated with regulation of chondrocyte proliferation and maturation. Therefore, when the cyclic mechanical forces are applied to the bottom of the Flexercell dishes, the cells are exposed to fluid flow stress,[88,89] which may consequently up-regulate the expression of PTHrP mRNA through a signal transduction pathway via the mobilization of Ca2+.
Meanwhile, Motokawa et al.[90] examined the influences of Gd3+ (gadolinium), an S-A channel inhibitor, and nifedipine as an L-type calcium channel blocker for the expression of VEGF and M-CSF in osteoblastic cells with mechanical stimuli. Gadolinium treatment reduced the amount of mRNA and protein concentration, but the nifedipine had no effect. These findings suggest that cyclic tensile forces increase the expression of VEGF and M-CSF in osteoblastic MC3T3-E1 cells via S-A channel. They explained these findings by a hypothesis that the mechanical stress-activated cellular mechanotransducer such as mechanosensitive ion channel, cytoskeleton, and integrins. S-A channel is a membrane stretch-activated ionic channel, which is localized in osteoblast-like cells.[91] Naruse and Sokabe[92] showed that stretching cellular membranes increased intracellular Ca2+ concentration in human umbilical endothelial cells, and that the Ca2+ response disappeared when extracellular Ca2+ was removed or treated with Gd3+ which is a potent blocker for the S-A channel. It was also demonstrated that cell orientating and elongating responses of cultured endothelial cells to cyclic stretch were inhibited by the removal of external Ca2+ or by adding Gd3+.[93] These findings suggest that cell orientating and elongating are mediated by Ca2+ permeable S-A channels that exist on the membrane of endothelial cells. Recently, the eukaryotic gene (Mid1) encoding S-A channel was identified from yeast. It was revealed that Mid1 acts as a calcium-permeable, cation-selective stretch-activated channel.[94] Thus, it may be confirmed that mechanical stretch to osteoblastic MC3T3-E1 cells might cause them to express VEGF and M-CSF mediated by the S-A channel, indicating that the signal transmission of mechanical stimuli to osteoblasts is different from that to chondrocytes, elucidated previously by Tanaka et al.[88]
Recently, Okamoto et al.[95] examined a signaling pathway from excessive mechanical stimuli to cellular and molecular changes and clarified that excessive mechanical stress activates integrin αV and affect the signaling pathway through phosphorylation of FAK, ERK and NF-κB in chondrocytes. Moreover, they suggested that the signaling pathway may modulate the cartilage turnover by regulating the expression of COX-2, IL-1 β, TNF-α, MMP3 and MMP13, although these findings may require further investigation in detail in near future.
In conclusion, on the basis of a series of researches, induction mechanisms of degenerative changes in articular cartilage and PCR regarded as TMJ-OA are depicted schematically in Figure 15.[96] An induction of large compressive stresses in the anterior and lateral areas on the condyle was recognized as an initial factor. Increase of friction at the articular surface was indicated as a cause of the larger stress and the relevant disk displacement. Excessive stresses on the condyle induced a decrease in the thickness of cartilage layers and an increase in the numbers of clast cells. Degenerative changes in the condylar cartilage and the expression of bone resorption-related factor were also observed. In a biochemical aspect, excessive mechanical stimuli, irrespective of compressive or tensile one, induced HA fragmentation, expression of proinflammatory cytokines and VEGF, an imbalance between MMPs and TIMPs, all of which are assumed to induce lower resistance to external stimuli and degenerative changes leading to bone and cartilage resorption. Furthermore, condylar resorption affects intra-articular mechanical environment which further induce degenerative changes in a progressive manner. If such a sequence is interrupted by an appropriate therapeutic system with an aid of sufficient host remodeling capacity, a functional and adaptive remodeling may be achieved,[1,17] as noted in Figure 15. Such therapeutic cues for TMJ-OA will be explained later in the actual case with PCR before treatment and adaptive repair of the damaged condyles after treatment.
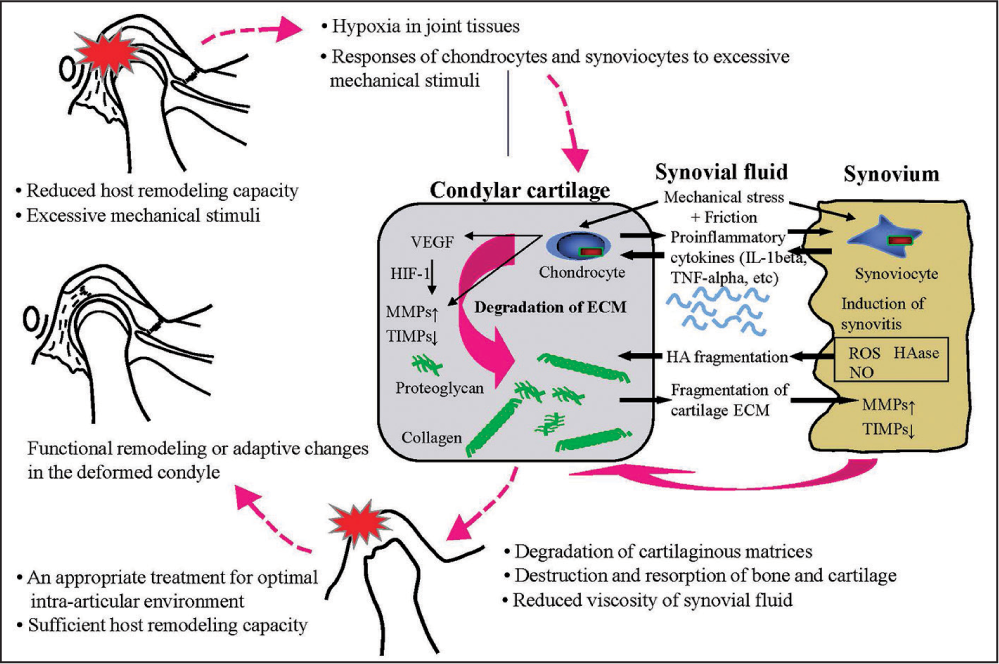
- Induction mechanisms of degenerative changes in articular cartilage and progressive condylar resorption regarded as temporomanidular joint-osteoarthritis
CLINICAL IMPLICATION FOR THE TREATMENT OF TEMPOROMANDIBULAR JOINT DISORDERS
Temporomandibular joint disorders have been regarded as one of the important diseases in dentistry and orthodontics. Among TMDs, internal derangement of the TMJ is the most prevalent in adolescent subjects.[7] It is emphasized that a certain type of malocclusion with a lack in occlusal stability produces condylar displacement in the TMJ space, and then disk displacement is induced as the condyle occupies concentric position.[11,12] Another explanation for TMJ internal derangement, derived from biomechanical studies on joint friction and synovial lubrication, is that pathologic changes in the TMJ space generate reduced viscosity of synovial fluid and greater friction, which finally induce disk displacement in TMJ internal derangement.[30,32]
For the treatment of TMJ-OA, we firstly have to perform appropriate and precise examinations enough for differential diagnosis.[7,97] In addition to the conventional examinations, a highly advanced biochemical examination of urinary bone resorption markers (pyridinoline and deoxypyridinoline) has recently been used for the detection of bone or cartilage destruction in TMJ-OA.[98,99] During a series of treatment after differential diagnosis, we have to achieve TMJ unloading or the biomechanical equilibrium by means of condylar repositioning, if indicated, and the subsequent occlusal reconstruction without producing adverse influences on TMJ structures and functions.[2,100] Treatment of TMD cases with myalgia of the masticatory muscles, disk displacement with reduction (ADDW) and TMJ-OA is shown below for better understanding the therapeutic system of TMDs with different intra-articular pathologic problems [Figure 16].
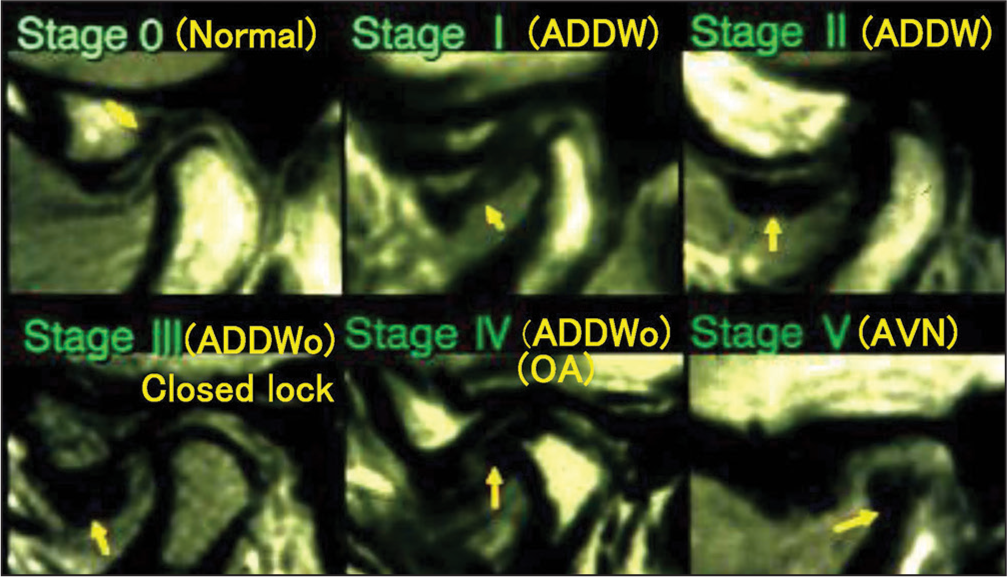
- Classification of intra-articular pathologic stages in temporomanidular joint-internal derangement defined by Wilkes
The first case is a 25-year-old male with neck and masticatory muscle tenderness and discomfort. He had Angle Class I molar relation with 1.0 mm overjet and 0.5 mm overbite. Maxillo-mandibular relation was Skeletal 3 and lateral shift of the mandible was 2.5 mm to the right. Condylar movement was somewhat irregular and restricted. As a result of functional and imaging examinations, the TMJs on both sides were diagnosed as asymptomatic excluding muscle disorders [Figure 17]. It is thus speculated that muscle disorders are due to unstable occlusion with right posterior cross-bite.
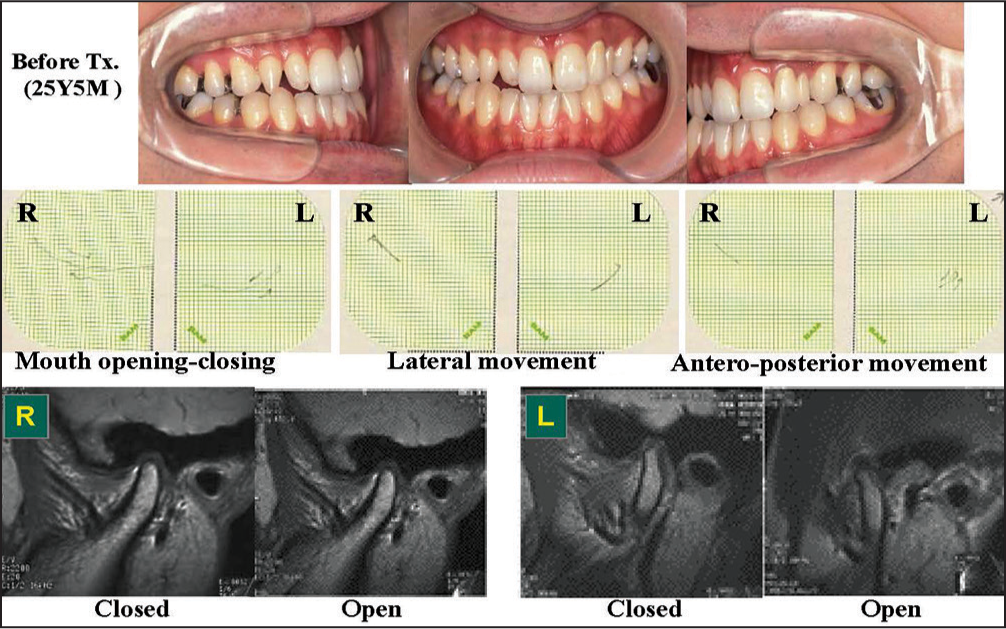
- Treatment of a patient with myalgia of the masticatory muscles top: Occlusion before treatment, middle: Condylar movement trajectory to show limited movement of the condyle, Bottom: Magnetic resonance images to show normal status of intra-articular pathology with articular disk on the condyle
After the use of stabilization splint, muscle tenderness was almost eliminated, demonstrating that the muscle disorder is an occlusion-related symptom. Occlusal reconstruction was initiated with lateral expansion of the upper dentition followed by orthodontic teeth alignment with multi-bracket appliances [Figure 18].
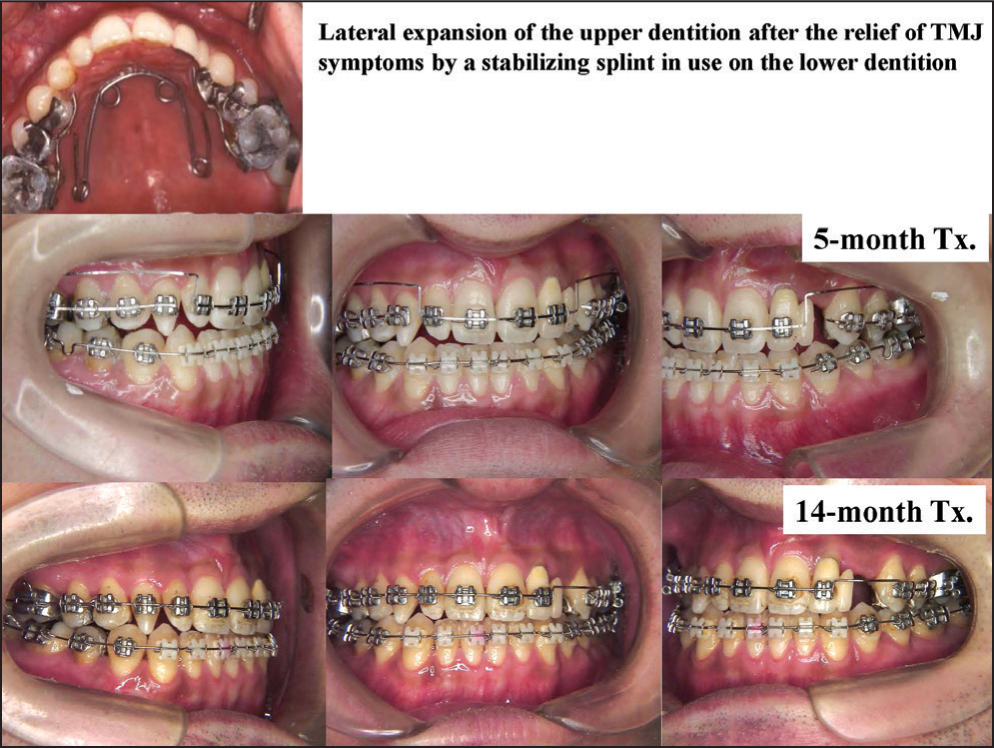
- Splint therapy followed by occlusal reconstruction with multi-bracket appliances
Stable occlusion was achieved. Then, prosthodontic treatment was carried out for the missing left upper canine area. After the treatment, condylar movement became much smoother and more uniform on both sides than before treatment [Figure 19].

- Occlusion after orthodontic (top) and prosthodontic (middle) treatments and smooth condylar movement (bottom) achieved by a series of orthodontic and prosthodontic treatments
Changes in the masticatory muscle activity are shown in Figure 20. Muscle activity became greater in association with better-balanced occlusal force distribution achieved by orthodontic tooth alignment. Subsequently, the muscle tenderness was eliminated.
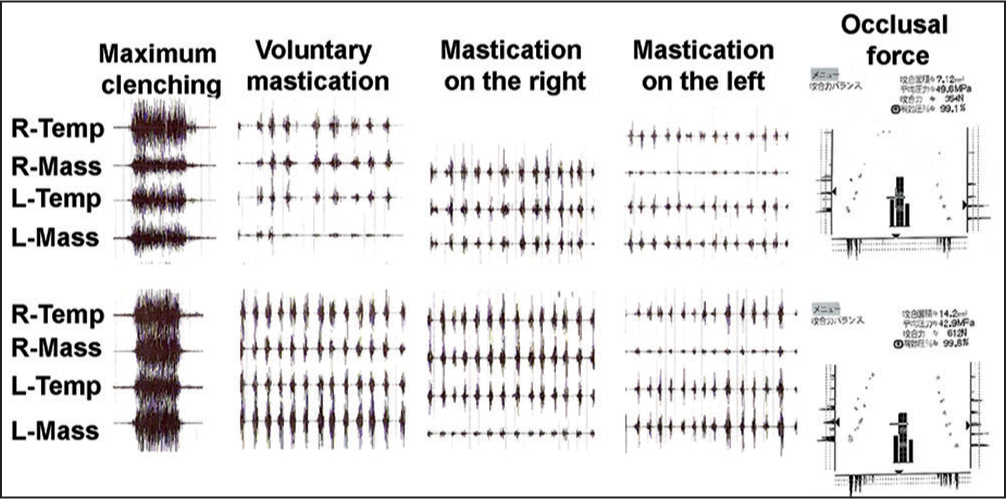
- Evaluation of masticatory muscle activity and occlusal contact and force before (a) and after treatment (b) masticatory muscle activity became greater in association with better balanced occlusal force distribution produced by orthodontic occlusal reconstruction in a patient with myalgia of the masticatory muscles
It is shown from this case that occlusal discrepancy from malocclusion induces imbalance of muscle activity leading to muscle tenderness and disorders, which in turn can be eliminated by optimal occlusion achieved by orthodontic treatment. Thus, it is demonstrated that orthodontic treatment can become a significant contributing factor to the correction of TMD.
The second case is a 34-year-old female with severe deep bite. Molar relation was angle Class II on the right and Class I on the left. Overjet and overbite were 3.0 and 7.5 mm, respectively [Figure 21]. Anteroposterior relation of the maxilla and mandible was skeletal 2, as denoted by the ANB angle of 8.2°.
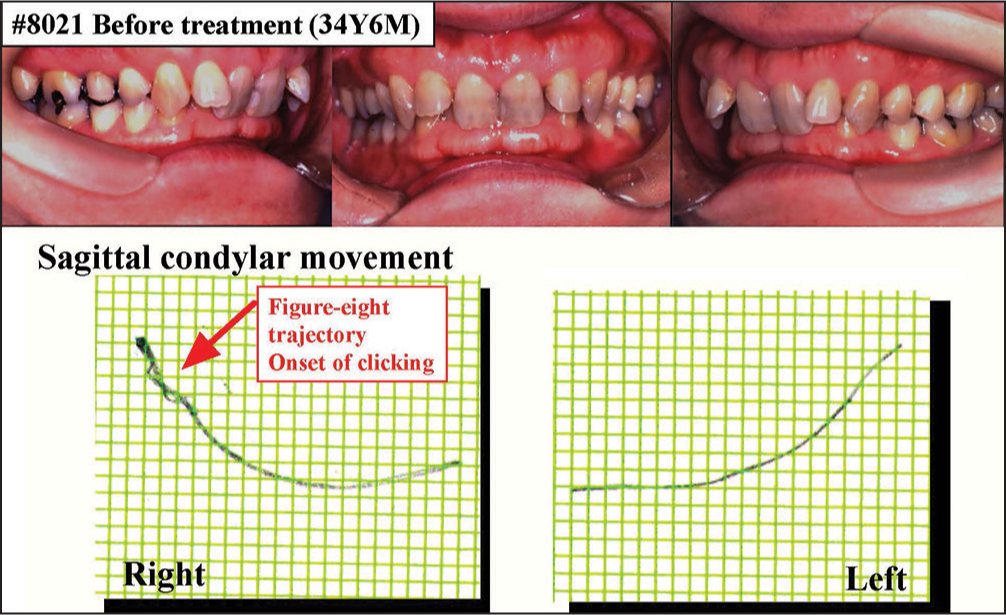
- Treatment of a patient with temporomanidular joint-internal derangement or anterior disk displacement with reduction (ADDW)
Temporomandibular joint pain and reciprocal clicking were observed as the TMD symptoms. The amount of maximum mouth opening was 42 mm. For the sagittal condylar movement, figure eight pattern or crossing of jaw opening and closing trajectories was detected in the right TMJ at the early stage of opening, which is regarded as an early-type figure eight pattern [Figure 21].
On the tomograms, posterior displacement of both condyles was clearly observed. On the magnetic resonance images (MRIs), anterior displacement of the disk with reduction was found in the right TMJ [Figure 22].

- Posterior position of both condyles on lateral tomograms (left) and anterior displacement of right articular disk with reduction on magnetic resonance images (right)
From these findings, anterior displacement of the disk with reduction or stage I TMJ internal derangement was diagnosed. Furthermore, the TMD symptoms in this case were speculated due to limited anterior movement of the mandible produced by the extruded maxillary incisors, indicating a possible association between malocclusion and TMD, and an absolute necessity of orthodontic approach for the treatment in this case.
Treatment procedures are shown in Figure 23. Two by two technique with multi-bracket appliance was used with a stabilization type splint placed on the lower dentition. This approach aims to move the upper incisors forward and upward and to allow smooth movement of the distally-located mandible and condyle. Five months later, following the correction of severe deep bite and lingual inclination of the upper incisors, multi-bracket appliances were used for the leveling. An anterior repositioning splint was placed in use on the upper dentition to allow the flattening of the occlusal plane. Then, the posterior part of the splint was cut gradually to extrude the lower molars and to achieve the posterior bite in the splint-induced position. Finally, the splint was cut into a small one placed on the anterior region.

- Occlusal reconstruction with orthodontic treatment and splint in use (to be continued)
Seventeen months after initiating the treatment with a splint, the occlusion became stable or invariable even if the splint was not used.
Optimal and excellent occlusion has been achieved by 20-month treatment [Figure 23].
Condylar movement became much smoother for both TMJs than before, indicating the functional correction of mandibular movement and repositioning of the disk and condyle in the TMJ space. MRIs after treatment demonstrated the repositioning of the disks, occupying the normal position between the condyle and glenoid fossa [Figure 24].
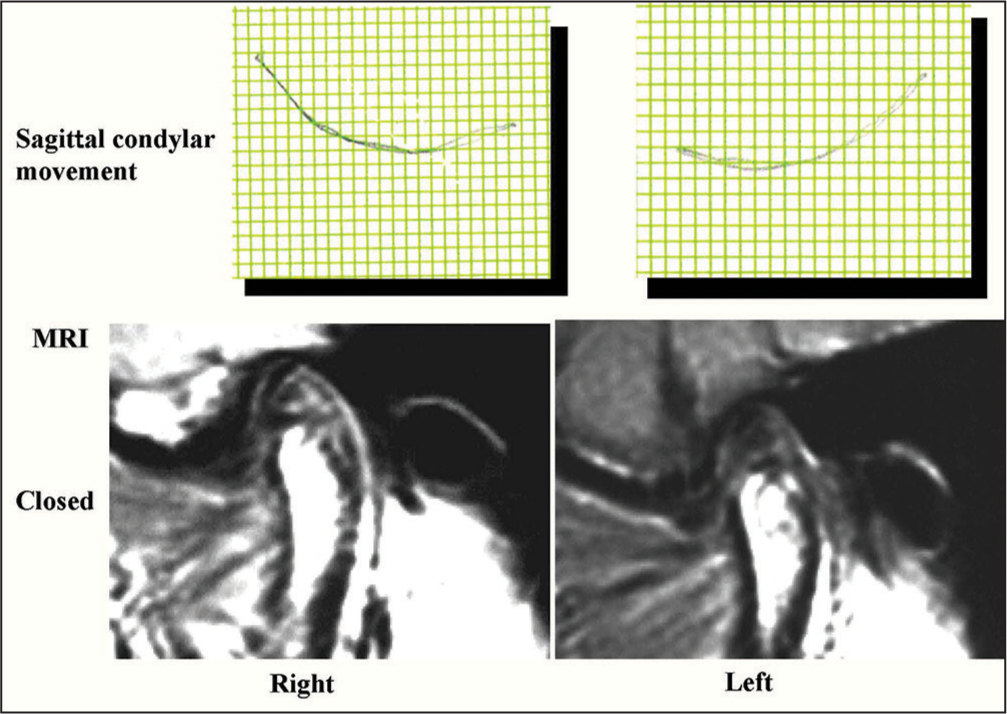
- Sagittal condylar movement and magnetic resonance images after treatment (36Y 2M)
The third case is a 21-year-old female with TMJ pain at the left TMJ, muscle tenderness at the left masseter and difficulty of jaw opening.[17] The amount of maximum mouth opening was 33.0 mm. She underwent splint therapy in a private dental clinic.
Molar relation was Angle Class II and overjet and overbite were 6.0 mm and −3.0 mm. Open bite was found at the anterior to the premolar region [Figure 25].
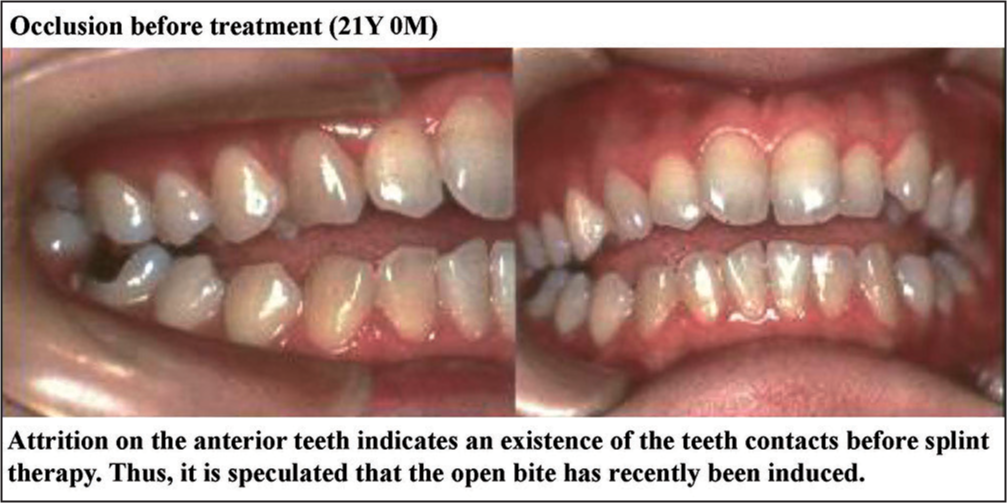
- Treatment of a patient with temporomanidular joint-osteoarthritis or anterior displacement of articular disk without reduction
For both TMJs, deformity of the condyle was observed. In particular, severe flattening was observed on the anterior surface of the left condyle. On the MRIs, anterior displacement of the disk without reduction and disk deformity were observed for both TMJs [Figure 26].
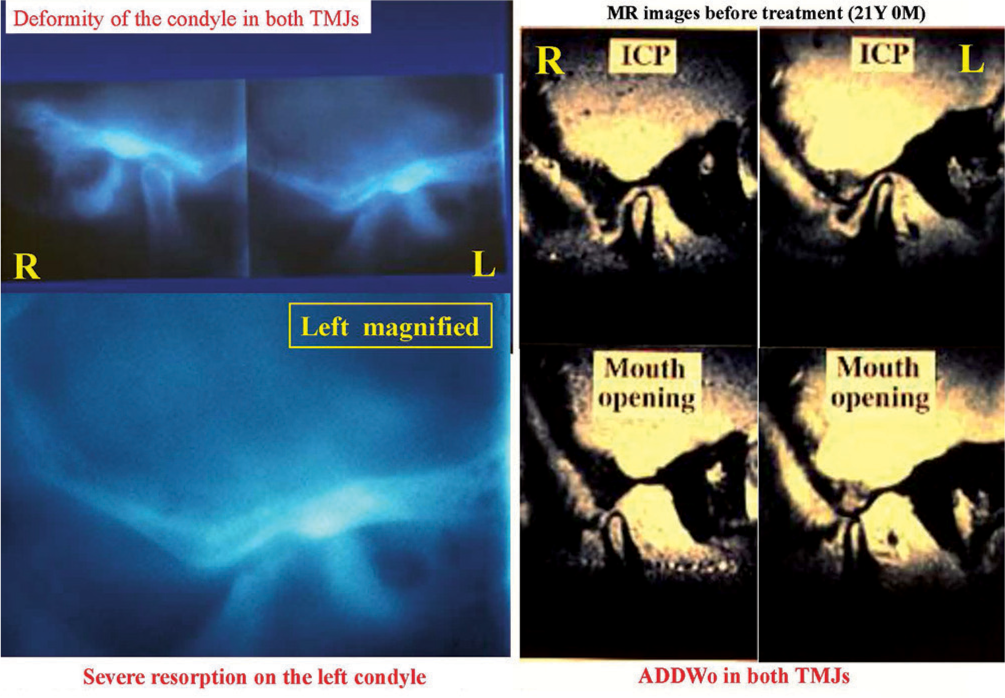
- Severe resorption of left condyle on lateral tomograms (left) and anterior displacement of articular disk without reduction on magnetic resonance images (right)
From these examinations, this case was diagnosed as ADDWo or stage IV internal derangement with TMJ-OA. According to the diagnosis, posterior bite splint was first used with manipulation to the TMJ to induce counter-clockwise rotation of the condyle and mandible followed by orthodontic occlusal reconstruction [Figure 27]. This approach aims to reduce TMJ loading in the anterior region where condylar resorption was prominent.
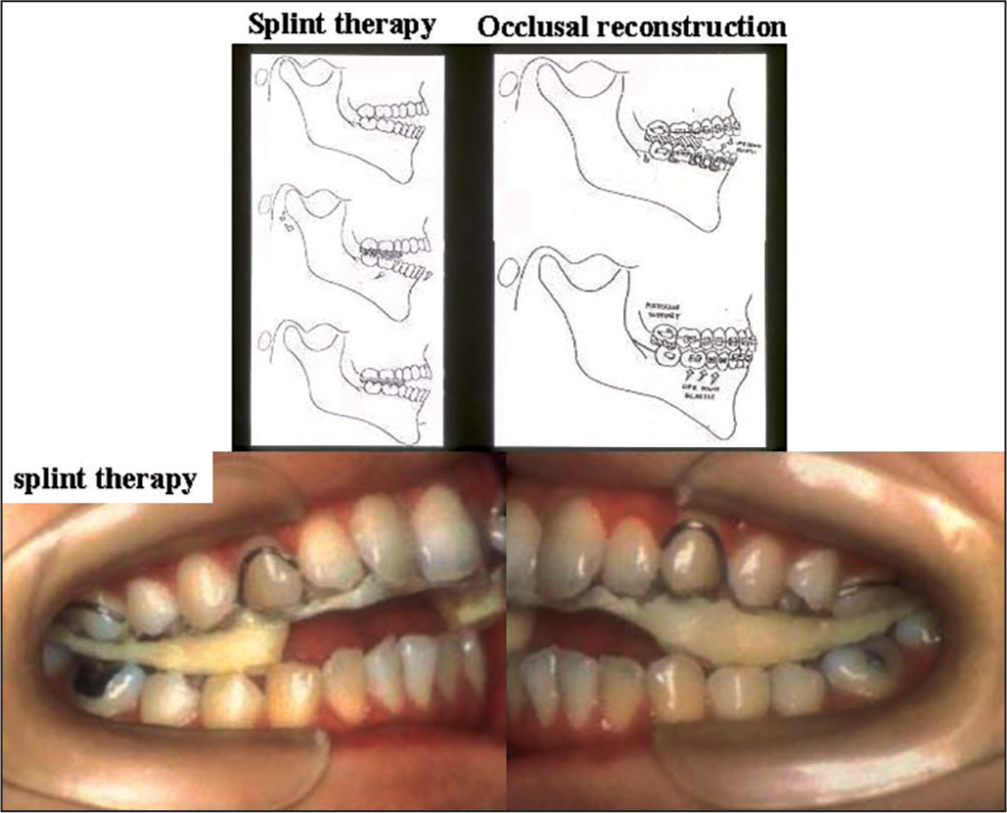
- A schematic illustration of splint therapy to eliminate temporomanidular joint pain and limited mouth opening and the following occlusal reconstruction with orthodontic approach
After the relief of TMJ pain and limited mouth opening, multi-bracket appliances were placed on the dentitions with a pair of mini splints on the molar regions. Furthermore, multi-loop edgewise archwire (MEAW) was employed to correct an open bite. Stable occlusion was obtained [Figure 28]. It is noted that adaptive changes are generated on the surfaces of condyle on both sides [Figure 29]. However, disk repositioning was not achieved as was expected before treatment.
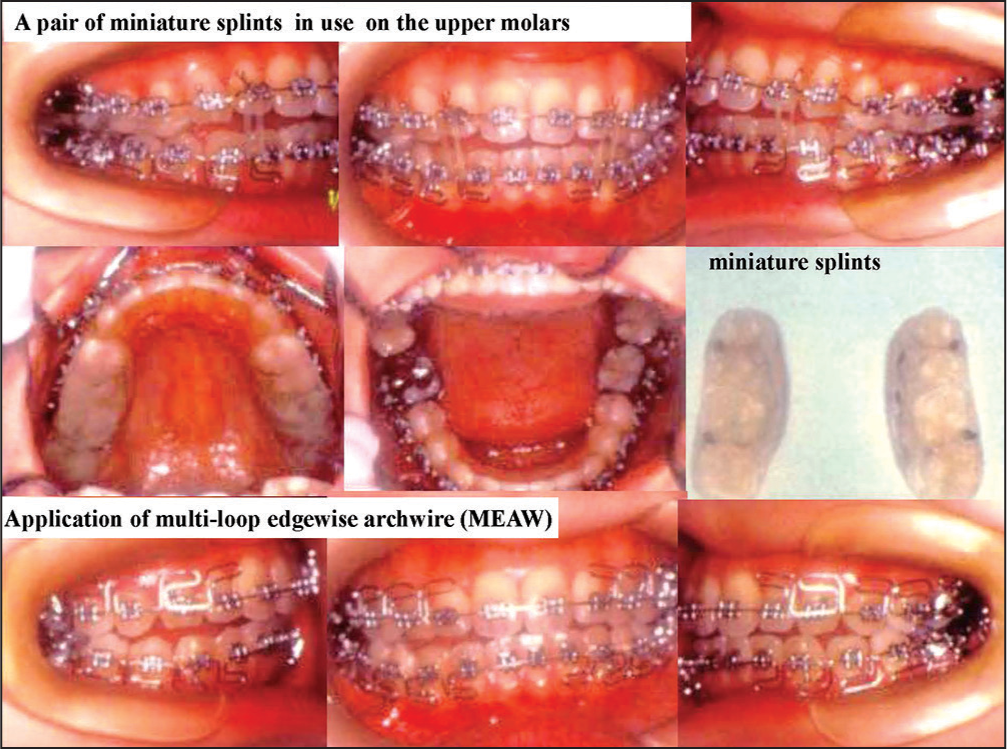
- Occlusal reconstruction with orthodontic treatment and splint in use

- Occlusion after treatment (upper) and adaptive repair of left condyle observed on lateral tomograms (lower)
It is surprising that the left condyle was reformed or exhibited unexpected adaptive responses [Figure 30]. It is demonstrated that the sable occlusion with orthodontic alignment has produced biomechanical equilibrium in the TMJ and subsequently provided the condyle with a potential for adaptive or functional remodeling. It is also shown that TMDs can be treated by an integrated approach to conservative treatment with a splint, if required, and the subsequent orthodontic teeth alignment.
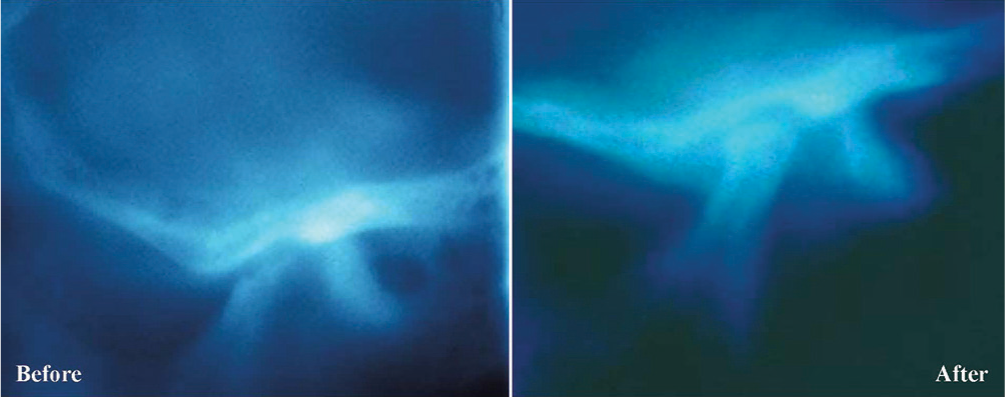
- Unexpected functional remodeling of the left condyle leading to the adaptive repair of left condyle, which exhibited prominent resorption before treatment
Finally, I would like to introduce a couple of pharmaceutical studies, which have recently been conducted in our research group, targeting on Celecoxib and Cilengitide, which are expected to prevent and/or repair degenerative changes in the mandibular condylar cartilage. First, Su et al.[101] examined the effect of Celecoxib on TMJ chondrocyte under high magnitude tensile stress in terms of PGE2 production, ECM-related gene expressions, and MMP-1 protein expression. As a result, high magnitude tensile stress-induced PGE2 level was abolished by Celecoxib. Celecoxib also diminished ECM-degradation and resorted ECM-synthesis gene expressions induced by high magnitude CTS. Furthermore, Celecoxib inhibited enhanced level of MMP-1 protein. It is thus shown that mechanical overload activates COX-2/PGE2 pathway and PGE2 exerts catabolic effects partially via EP4 receptor and that Celecoxib has an overall protective effect on TMJ-ECM metabolism by diminishing the catabolic effect of mechanical overload, supporting it a possible anti-OA drug for the treatment of TMJ-OA patients.
Then, Okamoto et al.[95] examined the effects of Cilengitide on gene expressions of pre-inflammatory factors by means of a quantitative real-time PCR. In addition, the mechano-transduction through integrin αV, proteins were examined by western blotting about phosphorylation of FAK, Akt, ERK and NF-κB. Treatment of Cilengitide significantly suppressed the gene expressions of COX-2, TNF-α, IL-1 β, MMP-3 and 13 dose-dependently. Protein levels of p-FAK, p-ERK and p-NF-κB were enhanced by excessive mechanical stress and suppressed dose-dependently by treatment with Cilengitide while total FAK and p-Akt were not affected significantly. From these findings, it is thus suggested that excessive mechanical stress activates integrin αV and affect the signaling pathway through phosphorylation of FAK, ERK and NF-κB in chondrocytes. Moreover, this signaling pathway may modulate the cartilage turnover by regulating the expression of COX-2, IL-1 β, TNF-α, MMP3 and MMP13. Moreover, it is highly emphasized that Cilengitide has a potential availability for the treatment of TMJ-OA by intercepting the attachment of chondrocytes and ECM which receive excessive mechanical stress.
References
- Progressive mandibular retrusion: Idiopathic condylar resorption. Part I and II. Am J Orthod Dentofacial Orthop. 1996;110:8-15. 117-27
- [Google Scholar]
- Relationship between orthodontic treatment, condylar position, and internal derangement in the temporomandibular joint. Am J Orthod Dentofacial Orthop. 1992;101:48-53.
- [Google Scholar]
- Reconsideration of the TMJ condylar position during internal derangement: Comparison between condylar position on tomogram and degree of disk displacement on MRI. Cranio. 1999;17:93-100.
- [Google Scholar]
- Associations between occlusal characteristics and signs and symptoms of TMJ dysfunction in children and young adults. Am J Orthod Dentofacial Orthop. 1987;92:467-77.
- [Google Scholar]
- Temporomandibular pain-dysfunction and occlusal relationships. Angle Orthod. 1973;43:136-53.
- [Google Scholar]
- Association of TMJ Pathology with Joint Function and Craniofacial Morphology in Orthodontic Patients with TMJ Internal Derangement. Ph.D. Thesis [in Japanese] thesis
- [Google Scholar]
- Prevalence of temporomandibular joint disorders in adult students: Evaluation of the intraarticular pathologic status by use of MRI. J Jpn Soc TMJ. 1998;10:335-47. [in Japanese with English abstract]
- [Google Scholar]
- Relation of pathologic status of TMJ and craniofacial morphology in patients with jaw deformities. Jpn J Jaw Deform. 1998;8:57-66. [in Japanese with English abstract]
- [Google Scholar]
- Association between malocclusion and temporomandibular disorders in orthodontic patients before treatment. J Orofac Pain. 1993;7:156-62.
- [Google Scholar]
- Association of condylar position with anterior disk displacement and disk morphology in patients with internal derangement of the TMJ: A study with axially corrected sagittal tomography and MRI. J Jpn Soc TMJ. 1997;9:439-49. [in Japanese with English abstract]
- [Google Scholar]
- Role of condylar position in TMJ dysfunction-pain syndrome. J Prosthet Dent. 1979;42:597.
- [Google Scholar]
- Craniofacial morphology in patients with anterior displacement of the temporomandibular joint disk without reduction. J Jpn Soc TMJ. 1994;6:54-68. [in Japanese with English abstract]
- [Google Scholar]
- Internal derangements of the temporomandibular joint. Pathological variations. Arch Otolaryngol Head Neck Surg. 1989;115:469-77.
- [Google Scholar]
- Internal derangement of the temporomandibular joint: Radiologic staging with clinical, surgical, and pathologic correlation. Magn Reson Imaging. 1989;7:495-515.
- [Google Scholar]
- Growth changes and orthodontic treatment in a patient with condylolysis. Am J Orthod Dentofacial Orthop. 1992;102:295-301.
- [Google Scholar]
- An adult case of TMJ osteoarthrosis treated with splint therapy and the subsequent orthodontic occlusal reconstruction: Adaptive change of the condyle during the treatment. Am J Orthod Dentofacial Orthop. 2000;118:566-71.
- [Google Scholar]
- Three-dimensional finite element analysis of the human temporomandibular joint disc. J Biomech. 2000;33:307-16.
- [Google Scholar]
- Three-dimensional finite element analysis of the cartilaginous structures in the human temporomandibular joint. J Dent Res. 2001;80:1913-8.
- [Google Scholar]
- A study of the control of disc movement within the temporomandibular joint using the finite element technique. J Oral Maxillofac Surg. 1996;54:1431-7.
- [Google Scholar]
- Biomechanical response of retrodiscal tissue in the temporomandibular joint under compression. J Oral Maxillofac Surg. 2002;60:546-51.
- [Google Scholar]
- Stress analysis in the TMJ during jaw opening by use of a three-dimensional finite element model based on magnetic resonance images. Int J Oral Maxillofac Surg. 2001;30:421-30.
- [Google Scholar]
- A three-dimensional finite element model of the mandible including the TMJ and its application to stress analysis in the TMJ during clenching. Med Eng Phys. 1994;16:316-22.
- [Google Scholar]
- Stress distributions in the TMJ during clenching in patients with vertical discrepancies of the craniofacial complex. J Orofac Pain. 1995;9:153-60.
- [Google Scholar]
- The temporomandibular joint. A morphologic study on a human autopsy material. Acta Odontol Scand. 1971;29:349-84.
- [Google Scholar]
- Topographical distribution of sulphated glycosaminoglycans in human temporomandibular joint disks. A histochemical study of an autopsy material. J Oral Pathol. 1976;5:265-76.
- [Google Scholar]
- The elastic modulus of the temporomandibular joint disc from adult dogs. J Dent Res. 1991;70:1545-8.
- [Google Scholar]
- In vitro measurement of the stress-distribution properties of the pig temporomandibular joint disc. Arch Oral Biol. 1994;39:439-48.
- [Google Scholar]
- The influence of loading time and lubricant on the friction of articular cartilage. Proc Inst Mech Eng H. 1996;210:109-19.
- [Google Scholar]
- Influence of friction at articular surfaces of the temporomandibular joint on stresses in the articular disk: A theoretical approach with the finite element method. Angle Orthod. 2003;73:319-27.
- [Google Scholar]
- Sliding friction analysis of phosphatidylcholine as a boundary lubricant for articular cartilage. Proc Inst Mech Eng H. 1993;207:59-66.
- [Google Scholar]
- The frictional coefficient of the temporomandibular joint and its dependency on the magnitude and duration of joint loading. J Dent Res. 2004;83:404-7.
- [Google Scholar]
- A theoretical formulation for boundary friction in articular cartilage. J Biomech Eng. 1997;119:81-6.
- [Google Scholar]
- Glycosaminoglycans and glycoproteins in synovial fluid In: Balazs EA, Jeanloz RW, eds. The Amino Sugars. The Chemistry and Biology of Compounds Containing Amino Sugars. New York: Academic Press; 1965. p. :229-50.
- [Google Scholar]
- Role of chondroitin sulfate-hyaluronan interactions in the viscoelastic properties of extracellular matrices and fluids. Biochim Biophys Acta. 1998;1380:1-9.
- [Google Scholar]
- Highly viscous sodium hyaluronate and joint lubrication. Int Orthop. 2002;26:116-21.
- [Google Scholar]
- Influence of additive hyaluronic acid on the lubricating ability in the temporomandibular joint. J Biomed Mater Res A. 2004;70:149-53.
- [Google Scholar]
- The disc of the human temporomandibular joint: Design, function and failure. J Oral Rehabil. 1985;12:279-93.
- [Google Scholar]
- Intraarticular pressure in the functioning human temporomandibular joint and its alteration by uniform elevation of the occlusal plane. J Oral Maxillofac Surg. 1994;52:671-9.
- [Google Scholar]
- The behaviour of collagen fibres in stress relaxation and stress distribution in the jaw-joint disc of rabbits. Arch Oral Biol. 1996;41:1039-52.
- [Google Scholar]
- Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech. 2006;39:312-22.
- [Google Scholar]
- Dynamic shear properties of the temporomandibular joint disc. J Dent Res. 2003;82:228-31.
- [Google Scholar]
- Viscoelastic properties of canine temporomandibular joint disc in compressive load-relaxation. Arch Oral Biol. 1999;44:1021-6.
- [Google Scholar]
- Viscoelastic material model for the temporomandibular joint disc derived from dynamic shear tests or strain-relaxation tests. J Biomech. 2007;40:2330-4.
- [Google Scholar]
- Biomechanical behavior of the temporomandibular joint disc. Crit Rev Oral Biol Med. 2003;14:138-50.
- [Google Scholar]
- Functional anatomy of the temporomandibular joint. A morphologic study on human autopsy material. Anat Embryol (Berl). 1991;183:89-95.
- [Google Scholar]
- A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch Oral Biol. 1989;34:749-57.
- [Google Scholar]
- An immunohistochemical study of the localization of biglycan, decorin and large chondroitin-sulphate proteoglycan in adult rat temporomandibular joint disc. Arch Oral Biol. 1998;43:889-98.
- [Google Scholar]
- Effects of enzymatic degradation after loading in temporomandibular joint. J Dent Res. [in press]
- [Google Scholar]
- Influences of vertical occlusal discrepancies on condylar responses and craniofacial growth in growing rats. Angle Orthod. 1999;69:356-64.
- [Google Scholar]
- The role of compressive forces in the normal maturation of the condylar cartilage in the rat. Acta Anat (Basel). 1977;97:351-60.
- [Google Scholar]
- Condylar cartilage response to continuous mandibular displacement in the rat. Angle Orthod. 1986;56:49-57.
- [Google Scholar]
- Quantitative analysis of temporomandibular joint adaptations to protrusive function. Am J Orthod. 1979;76:593-611.
- [Google Scholar]
- An experimental study of increased vertical dimension in the growing face. Am J Orthod. 1977;71:382-95.
- [Google Scholar]
- Jaw-muscle activity changes after the induction of osteoarthrosis in the temporomandibular joint by mechanical loading. J Orofac Pain. 2008;22:153-62.
- [Google Scholar]
- Vascular endothelial growth factor plays an important autocrine/ paracrine role in the progression of osteoarthritis. Histochem Cell Biol. 2005;123:275-81.
- [Google Scholar]
- The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res. 2002;17:284-92.
- [Google Scholar]
- Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685-93.
- [Google Scholar]
- Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542-9.
- [Google Scholar]
- Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604-13.
- [Google Scholar]
- Osteoarthrosis of the hip in women and its relation to physical load at work and in the home. Ann Rheum Dis. 1997;56:293-8.
- [Google Scholar]
- Matrix loss and synthesis following a single impact load on articular cartilage in vitro. Biochim Biophys Acta. 1997;1334:223-32.
- [Google Scholar]
- Detection of tumor necrosis factor alpha and interleukin-1 beta in the rheumatoid osteoarthritic cartilage-pannus junction by immunohistochemical methods. Rheumatol Int. 1993;13:77-82.
- [Google Scholar]
- Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84:678-85.
- [Google Scholar]
- Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem (Tokyo). 1999;125:966-75.
- [Google Scholar]
- Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol. 1999;154:1755-62.
- [Google Scholar]
- Induction of CD44 and MMP expression by hyaluronidase treatment of articular chondrocytes. J Biochem. 2004;135:567-75.
- [Google Scholar]
- The metabolism of hyaluronan in cultured rabbit growth plate chondrocytes during differentiation. Biochim Biophys Acta. 2005;1743:57-63.
- [Google Scholar]
- Proinflammatory cytokines regulate the gene expression of hyaluronic acid synthetase in cultured rabbit synovial membrane cells. Connect Tissue Res. 2001;42:187-95.
- [Google Scholar]
- Hyaluronidase expression in cultured growth plate chondrocytes during differentiation. Cell Tissue Res. 2004;318:335-42.
- [Google Scholar]
- The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol. 2000;79:601-9.
- [Google Scholar]
- Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996;109(Pt 4):713-26.
- [Google Scholar]
- Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181-9.
- [Google Scholar]
- Cytoskeleton regulates expression of genes for transforming growth factor-beta 1 and extracellular matrix proteins in dermal fibroblasts. J Cell Physiol. 1997;172:192-9.
- [Google Scholar]
- Divergent regulation by growth factors and cytokines of 95 kDa and 72 kDa gelatinases and tissue inhibitors or metalloproteinases-1,-2, and-3 in rabbit aortic smooth muscle cells. Biochem J. 1996;315(Pt 1):335-42.
- [Google Scholar]
- Factors related to degradation of articular cartilage in osteoarthritis: A review. Semin Arthritis Rheum. 1998;27:392-9.
- [Google Scholar]
- Expression of superficial zone protein in mandibular condyle cartilage. Osteoarthritis Cartilage. 2006;14:807-13.
- [Google Scholar]
- Effects of mechanical stimuli on the synthesis of superficial zone protein in chondrocytes. J Biomed Mater Res A. 2010;92:801-5.
- [Google Scholar]
- Down-regulation of chondrocyte aggrecan and type-II collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res. 1999;17:836-42.
- [Google Scholar]
- Effect of compressive forces on extracellular matrix in rat mandibular condylar cartilage. J Bone Miner Metab. 2003;21:276-86.
- [Google Scholar]
- Chondrocyte differentiation is modulated by frequency and duration of cyclic compressive loading. Ann Biomed Eng. 2001;29:476-82.
- [Google Scholar]
- Accelerated chondrogenesis of the rabbit cranial base growth plate by oscillatory mechanical stimuli. J Bone Miner Res. 2002;17:1843-50.
- [Google Scholar]
- Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;18:391-9.
- [Google Scholar]
- Influences of mechanical stimuli on the endochondral ossification of condylar and growth plate chondrocytes: differences in the effects depending on the varying frequencies and property. Ph.D. Thesis [in Japanese] thesis
- [Google Scholar]
- Development and experimental validation of a fluid/structure-interaction finite element model of a vacuum-driven cell culture mechanostimulus system. Comput Methods Biomech Biomed Engin. 2000;3:65-78.
- [Google Scholar]
- Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: Ion-channel dependent transduction of matrix deformation signals. Exp Cell Res. 2000;256:383-91.
- [Google Scholar]
- Cyclic mechanical strain regulates the PTHrP expression in cultured chondrocytes via activation of the Ca2+ channel. J Dent Res. 2005;84:64-8.
- [Google Scholar]
- Mechanisms contributing to fluid-flow-induced Ca2+ mobilization in articular chondrocytes. J Cell Physiol. 1999;180:402-8.
- [Google Scholar]
- Effects of cyclic tensile forces on the expression of vascular endothelial growth factor (VEGF) and macrophage-colony-stimulating factor (M-CSF) in murine osteoblastic MC3T3-E1 cells. J Dent Res. 2005;84:422-7.
- [Google Scholar]
- Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR 106) FEBS Lett. 1989;251:17-21.
- [Google Scholar]
- Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am J Physiol. 1993;264:C1037-44.
- [Google Scholar]
- Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol. 1998;274:H1532-8.
- [Google Scholar]
- Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science. 1999;285:882-6.
- [Google Scholar]
- Functional mechanism of Integrin as mechanoreceptor in the metabolism of chondrocytes under excessive mechanical stress. In: Poster Presentation at the 47th Annual Meeting of Korean Association of Orthodontists.
- [Google Scholar]
- Degenerative changes of articular cartilage in association with mechanical stimuli. Jap Dent Sci Rev. 2008;44:38-47.
- [Google Scholar]
- Diagnostic accuracy of sagittal condylar movement patterns for identifying internal derangement of the temporomandibular joint. J Orofac Pain. 1997;11:222-31.
- [Google Scholar]
- Changes in urinary bone resorption markers (pyridinoline, deoxypyridinoline) resulting from experimentally-induced osteoarthritis in the temporomandibular joint of rats. Cranio. 2003;21:38-45.
- [Google Scholar]
- Utility of urinary pyridinoline and deoxypyridinoline ratio for diagnosis of osteoarthritis at temporomandibular joint. J Oral Pathol Med. 2004;33:218-23.
- [Google Scholar]
- Preventing adverse effects on the temporomandibular joint through orthodontic treatment. Am J Orthod Dentofacial Orthop. 1987;91:493-9.
- [Google Scholar]
- Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthritis Cartilage. 2014;22:845-51.
- [Google Scholar]






